
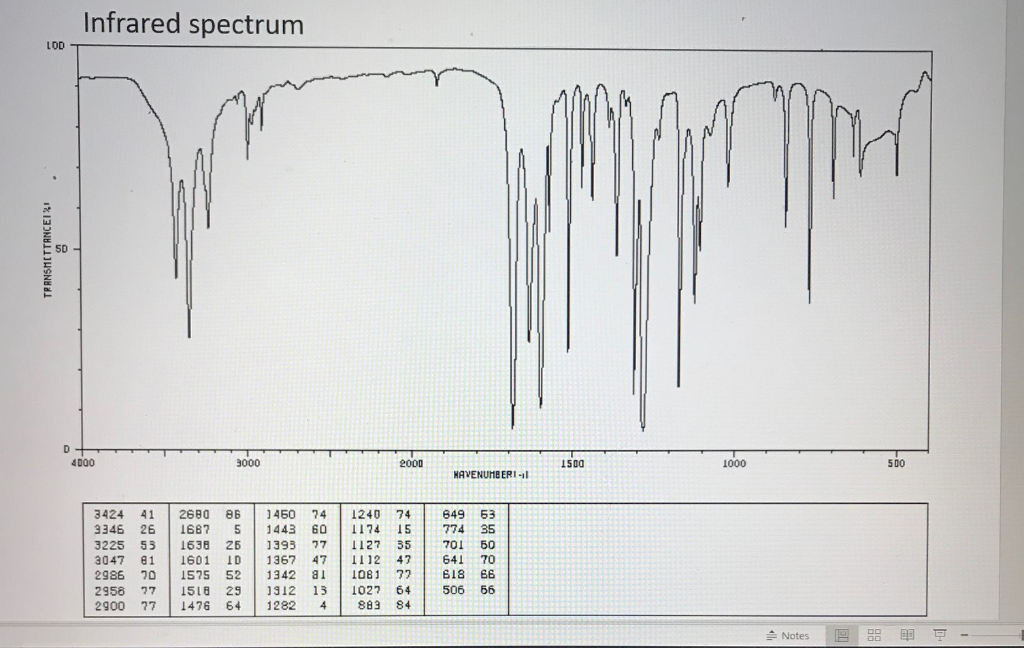
Thus a sample that did not absorb at all would record a horizontal line at 100% transmittance (top of the chart). The inverted display of absorption, compared with UV-Visible spectra, is characteristic. Further analysis (below) will show that this spectrum also indicates the presence of an aldehyde function, a phenolic hydroxyl and a substituted benzene ring. The gap in the spectrum between 700 & 800 cm -1 is due to solvent (CCl 4) absorption. The complexity of this spectrum is typical of most infrared spectra, and illustrates their use in identifying substances. An example of such a spectrum is that of the flavoring agent vanillin, shown below. Infrared spectrometers, similar in principle to the UV-Visible spectrometer described elsewhere, permit chemists to obtain absorption spectra of compounds that are a unique reflection of their molecular structure. Consequently, virtually all organic compounds will absorb infrared radiation that corresponds in energy to these vibrations. We must now recognize that, in addition to the facile rotation of groups about single bonds, molecules experience a wide variety of vibrational motions, characteristic of their component atoms. The mobile nature of organic molecules was noted in the chapter concerning conformational isomers. The covalent bonds in molecules are not rigid sticks or rods, such as found in molecular model kits, but are more like stiff springs that can be stretched and bent. Photon energies associated with this part of the infrared (from 1 to 15 kcal/mole) are not large enough to excite electrons, but may induce vibrational excitation of covalently bonded atoms and groups. The portion of the infrared region most useful for analysis of organic compounds is not immediately adjacent to the visible spectrum, but is that having a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*10 13 to 1.2*10 14 Hz. On the immediate high energy side of the visible spectrum lies the ultraviolet, and on the low energy side is the infrared. As noted in a previous chapter, the light our eyes see is but a small part of a broad spectrum of electromagnetic radiation.


 0 kommentar(er)
0 kommentar(er)
